Ozone is a bluish, colored gas that has a boiling point of -112 oC at atmospheric pressure; ozone can partially dissolve in water. At standard pressure and temperature, the solubility of ozone is thirteen times that of oxygen. The oxidation potential of 2.07 Volt proves that ozone is a strong oxidizer. Concentrated mixtures of ozone and oxygen that contain more than 20% ozone can become explosive in both fluids and gases. In commercial ozone generators these concentrations do not occur, because these cannot easily be generated. Ozone is fairly unstable in a watery solution; its half-life in water is about 20 minutes. In air, ozone has a half-life of 12 hours, which makes the stability of ozone in air superior.
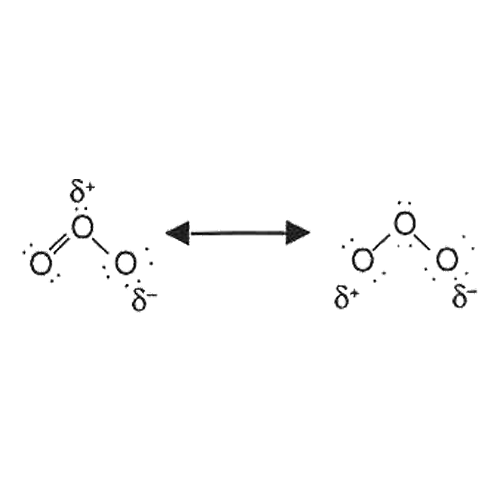
The symbols d+ en d- shows that ozone molecules are short of electrons on the locations where these signs occur. This means that ozone is a dipolar molecule. This causes ozone to have characteristic properties. Ozone reacts very selectively and is electrophilic.
| Symbol | O3 |
| Atomic weight | 48 |
| Melting point | -192,5 °C |
| Boiling point | -119,5 °C |
| Critical temperature | -12,1 °C |
| Critical pressure | 5460 kPa |
| Density | 2,14 kg O3/m3 bij 0 °C 1013 mbar |
| Relative density (in air) | 1,7 kg/m3 |
| Solubility | 570 mg/l bij 20 °C |
| Energy | 142,3 KJ/mol (34,15 kcal/mol) |
| Binding degree | 116 ° |
| Electrochemical potential | 2,07 volt |
| Occurence | Blueish gas, fluid dark blue |