
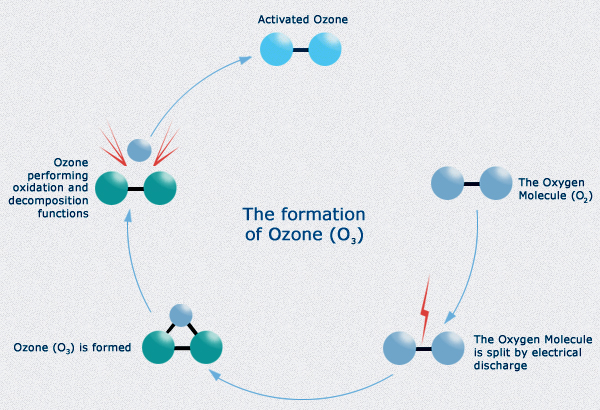
An electrical discharge splits an oxygen molecule into two oxygen atoms.
These highly reactive and unstable oxygen atoms combine with more oxygen molecules. This combination forms ozone.
Atmospheric ozone is mainly concentrated in the stratosphere, about 15-30 kilometers above the Earth's surface. The Earth's atmosphere is basically divided into several layers. The lowest region, the troposphere, extends from the earth's surface up to about 10 kilometers (Km) in altitude. The next layer, the stratosphere, continues from 10 km to about 50 km.
| Property | Ozone | vs. Oxygen |
| Molecular Formula: | O3 | O2 |
| Molecular Weight: | 48 g/mol | 32 g/mol |
| Smell: | - clothes after being outside on clothesline - photocopy machines - smell after lightning storms |
odorless |
| Color: | light blue | colorless |
| Boilint Point: | -111.3 deg C (-168.4 deg F) | -183 deg C (-297.4 deg F) |
| Density: | 2.141 kg/m3 (0.133 lb/ft3) | 1.429 kg/m3 |
| Electrochemical Potential: | 2.07 | 1.23 |
| Specific gravity @ STP (air = 1): | 1.612 | 1.105 |
| Solubility in Water (0-deg C): | 190 mg/l | 14.6 mg/l |
| CAS number | 10028-15-6 | 7782-44-7 |
| Odor Threshold: | 5-20 PPB (0.005 - 0.02 PPM) | odorless |